
Research Interests
Prof. Dr. D‘Alessandria’s lab research focus on basic and preclinical radiopharmacy/radiochemistry to develop new peptide and antibody-based radiotheranostic tracers and to bring these new tools in clinical application.
Our mission:
- To design, characterize and validate novel small agents and biomolecules radiolabeled with radioisotopes for non-invasive PET imaging and radiotherapy
- To decipher molecular profiles of inflammatory processes and cancer using nuclear imaging and plan new treatments with with endoradiotherapy
- To bring these agents and methodologies from bench into clinical application using GMP
- Educate and train students and scientists in onco-imaging, immunotherapy monitoring, inflammation imaging and radiotheranostics
Application - Open Positions
Currently, we do not have any open Postdoc or PhD positions. Applications from talented and motivated postdocs are always welcome. Please send your CV, list of publications and briefly describe your research interests and experience via email to Dr. D'Alessandria(link sends e-mail). Postdocs are expected to apply for stipends and fellowships. We will support you in the application process!
We are always looking forward to highly motivated students to conduct internships, Master theses and medical doctoral theses in our lab. If you are interested in an applied, interdisciplinary project, you are self-motivated, curious, organized and willing to spend a few months in the lab, please send a CV and motivation letter to Dr. D'Alessandria (link sends e-mail)via Email.
Short CV - Prof. Dr. Calogero D'Alessandria
Prof. Dr. Calogero D’Alessandria, PhD, is Head of Radiochemistry at the Department of Nuclear Medicine of the Technical University of Munich (TUM). Dr D’Alessandria graduated and received his PhD in “Physiopathology and Radioisotopical Imaging” at the University “Sapienza” in Rome, Italy. He joined the Department of Nuclear Medicine at TUM in 2007, when he overtook the responsibility of the Radiotherapy Unit.
Prof. Dr. D’Alessandria was trained in Radiopharmacy and Radiochemistry during his research fellows at the Nuclear Medicine Research Laboratory of Imperial Cancer Research Fund (ICRF) of Queen Mary University at St. Bartholomew´s Hospital, London, UK (head Prof. Stephen J. Mather) and at the Institut der Nuklearmedizin Radiologische Chemie Labor Kantonsspital, Basel, Switzerland (Head Prof. Helmut R. Mäcke). Dr. D’Alessandria’s lab research focus on design, development and validation of bifunctional chelators for Al18F-labeling, 89Zr-labeling, radiolabeled bio-molecules for T cell trafficking and homing visualization, and peptides for tumor detection, with high clinical translation potential, like probes for targeting the tumor microenvironment modulator Galectin-3 and chaperon molecules like Hsp70. He is interested also in targeted radionuclide therapy of cancer using Y-90, Lu-177, Bi-213 and Ac-225 labeled tracers.
Prof. Dr. D’Alessandria has published 53 papers in leading scientific journals including Cancer Research, Journal of Nuclear Medicine, European Journal of Nuclear Medicine and Molecular Imaging, Lancet Oncology, European Urology, Molecular Imaging and Biology and Theranostics.
Peer-reviewed
- Russelli L, De Rose F, Leone L, Reder S, Schwaiger M, D’Alessandria C*, Tei L*. A semi rigid novel hydroxamate AMPED-based ligand for 89Zr PET imaging.Molecules 2021; doi: 10.3390/molecules26195819.
- Feuerecker B, Chantadisai M, Allmann A, Tauber R, Allmann J, Steinhelfer L, Rauscher I, Wurzer A, Wester HJ, Weber WA, D'Alessandria C*, Eiber M*. Pre-therapeutic comparative dosimetry of 177Lu-rhPSMA-7.3 and 177Lu-PSMAI&T in patients with metastatic castration resistant prostate cancer (mCRPC). J Nucl Med. 2021 Sep 16:jnumed.121.262671.
- Gafita A, Calais J, Grogan TR, Hadaschik B, Wang H, Weber M, Sandhu S, Kratochwil C, Esfandiari R, Tauber R, Zeldin A, Rathke H, Armstrong WR, Robertson A, Thin P, D'Alessandria C, Rettig MB, Delpassand ES, Haberkorn U, Elashoff D, Herrmann K, Czernin J, Hofman MS, Fendler WP, Eiber M. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. 2021 Aug;22(8):1115-1125.
- Yusufi N, Wurzer A, Herz M, D'Alessandria C, Feuerecker B, Weber W, Wester HJ, Nekolla S, Eiber M. Comparative Preclinical Biodistribution, Dosimetry, and Endoradiotherapy in Metastatic Castration-Resistant Prostate Cancer Using 19F/177Lu-rhPSMA-7.3 and 177Lu-PSMA I&T. J Nucl Med. 2021 Aug 1;62(8):1106-1111.
- Birindelli G, Drobnjakovic M, Morath V, Steiger K, D'Alessandria C, Gourni E, Afshar-Oromieh A, Weber W, Rominger A, Eiber M, Shi K. Is Hypoxia a Factor Influencing PSMA-Directed Radioligand Therapy?-An In Silico Study on the Role of Chronic Hypoxia in Prostate Cancer. Cancers (Basel). 2021 Jul 8;13(14):3429.
- Chantadisai M, Buschner G, Krönke M, Rauscher I, Langbein T, Nekolla SG, Schiller K, Heck MM, Maurer T, Wurzer A, Wester HJ, D'Alessandria C, Weber W, Eiber M. Positive Predictive Value and Correct Detection Rate of 18F-rhPSMA-7 PET in Biochemically Recurrent Prostate Cancer Validated by Composite Reference Standard. J Nucl Med. 2021 Jul 1;62(7):968-974.
- Peplau E, De Rose F, Eichinger A, Reder S, Mittelhäuser M, Scafetta G, Schwaiger M, Weber WA, Bartolazzi A, D'Alessandria C, Skerra A. Effective rational humanization of a PASylated anti-galectin-3 Fab for the sensitive PET imaging of thyroid cancer in vivo. Sci Rep. 2021 Apr 1;11(1):7358.
- Rauscher I, Karimzadeh A, Schiller K, Horn T, D'Alessandria C, Franz C, Wörther H, Nguyen N, Combs SE, Weber WA, Eiber M. Detection efficacy of 18F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment. J Nucl Med. 2021 Mar 12:jnumed.120.260091. doi: 10.2967/jnumed.120.260091. Online ahead of print.
- Feuerecker B, Tauber R, Knorr K, Heck M, Beheshti A, Seidl C, Bruchertseifer F, Pickhard A, Gafita A, Kratochwil C, Retz M, Gschwend JE, Weber WA, D'Alessandria C*, Morgenstern A*, Eiber M*. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur Urol. 2021 Mar;79(3):343-350.
- Varasteh Z, De Rose F, Mohanta S, Li Y, Zhang X, Miritsch B, Scafetta G, Yin C, Sager HB, Glasl S, Gorpas D, Habenicht AJR, Ntziachristos V, Weber WA, Bartolazzi A, Schwaiger M, D'Alessandria C. Imaging atherosclerotic plaques by targeting Galectin-3 and activated macrophages using (89Zr)-DFO- Galectin3-F(ab')2 mAb. Theranostics. 2021 Jan 1;11(4):1864-1876.
- Gafita A, Heck MM, Rauscher I, Tauber R, Cala L, Franz C, D'Alessandria C, Retz M, Weber WA, Eiber M. Early Prostate-Specific Antigen Changes and Clinical Outcome After 177Lu-PSMA Radionuclide Treatment in Patients with Metastatic Castration-Resistant Prostate Cancer. J Nucl Med. 2020 Oct;61(10):1476-1483.
- Peplau E, De Rose F, Reder S, Mittelhäuser M, Scafetta G, Schwaiger M, Weber WA, Bartolazzi A, Skerra A, D'Alessandria C. Development of a Chimeric Antigen-Binding Fragment Directed Against Human Galectin-3 and Validation as an Immuno-Positron Emission Tomography Tracer for the Sensitive In Vivo Imaging of Thyroid Cancer. Thyroid. 2020 Sep;30(9):1314-1326.
- Russelli L, Martinelli J, De Rose F, Reder S, Herz M, Schwaiger M, Weber W, Tei L, D'Alessandria C. Room Temperature Al18 F Labeling of 2-Aminomethylpiperidine-Based Chelators for PET Imaging. ChemMedChem. 2020 Feb 5;15(3):284-292.
- Gafita A, Wang H, Tauber R, D'Alessandria C, Weber WA, Eiber M. Exceptional 4-year response to 177Lu-PSMA radioligand therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019 Sep;46(10):2212-2213.
- Mayer KE, Mall S, Yusufi N, Gosmann D, Steiger K, Russelli L, de Oliviera Bianchi H, Audehm S, Wagner R, Bräunlein, Stelzl A, Bassermann F, Weichert W, Weber W, Schwaiger M, D`Alessandria C, Krackhardt AM. T-cell functionality testing is highly relevant to develop novel immuno-tracers monitoring T cells in the context of immunotherapies and revealed CD7 as attractive target. Theranostics. 2018 Nov 28;8(21):6070-6087. doi: 10.7150/thno.27275.
- Karimi Ghodoosi E, D'Alessandria C, Li Y, Bartel A, Köhner M, Höllriegl V, Navab N, Eiber M, Li WB, Frey E, Ziegler S. The effect of attenuation map, scatter energy window width, and volume of interest on the calibration factor calculation in quantitative 177Lu SPECT imaging: Simulation and phantom study. Phys Med. 2018 Dec;56:74-80. doi: 10.1016/j.ejmp.2018.11.009.
- Heck MM, Tauber R, Schwaiger S, Retz M, D'Alessandria C, Maurer T, Gafita A, Wester HJ, Gschwend JE, Weber WA, Schwaiger M, Knorr K, Eiber M. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with 177Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2018 Nov 22. pii: S0302-2838(18)30873-X. doi: 10.1016/j.eururo.2018.11.016.
- Gafita A, Rauscher I, Retz M, Knorr K, Heck M, Wester HJ, D'Alessandria C, Weber WA, Eiber M, Tauber R. Early experience of rechallenge 177Lu-PSMA radioligand therapy after an initial good response in patients with mCRPC. J Nucl Med. 2018 Nov 15. pii: jnumed.118.215715. doi: 10.2967/jnumed.118.215715.
- De Rose F, Braeuer M, Braesch-Andersen S, Otto AM, Steiger K, Reder S, Mall S, Nekolla S, Schwaiger M, Weber WA, Bartolazzi A, D'Alessandria C. Galectin-3 targeting in thyroid orthotopic tumors opens new ways to characterize thyroid cancer. J Nucl Med. 2018 Oct 25. pii: jnumed.118.219105. doi: 10.2967/jnumed.118.219105.
- Stangl S, Tei L, De-Rose F, Reder S, Martinelli J, Sievert W, Shevtsov M, Öllinger R, Rad R, Schwaiger M, D’Alessandria C*, Multhoff G*. Preclinical evaluation of the Hsp70 peptide tracer TPP-PEG24-DFO[89Zr] for tumor-specific PET/CT imaging. Cancer Res. 2018 Nov 1;78(21): 6268-6281.
- Kletting P, Thieme A, Eberhardt N, Rinscheid A, D'Alessandria C, Allmann J, Wester HJ, Tauber R, Beer AJ, Glatting G, Eiber M. Modeling and predicting tumor response in radioligand therapy. J Nucl Med. 2019 Jan; 60 (1): 65-70. doi: 10.2967/jnumed.118.210377. Epub 2018 May 10.
- Autenrieth ME, Seidl C, Bruchertseifer F, Horn T, Kurtz F, Feuerecker B, D'Alessandria C, Pfob C, Nekolla S, Apostolidis C, Mirzadeh S, Gschwend JE, Schwaiger M, Scheidhauer K, Morgenstern A. Treatment of carcinoma in situ of the urinary bladder with an alpha-emitter immunoconjugate targeting the epidermal growth factor receptor: a pilot study. Eur J Nucl Med Mol Imaging. 2018 Apr 11. doi: 10.1007/s00259-018-4003-6. [Epub ahead of print]
- Begum NJ, Thieme A, Eberhardt N, Tauber R, D’Alessandria C, Beer AJ, Glatting G, Eiber M, Kletting P. The effect of total tumor volume on the biologically effective dose of tumor and kidneys for 177Lu-labelled PSMA peptides. J Nucl Med. 2018 Jun;59(6):929-933.
- Pfob CH, Eiber M, Luppa P, Maurer F, Maurer T, Tauber R, D'Alessandria C, Feuerecker B, Scheidhauer K, Ott A, Heemann U, Schwaiger M, Schmaderer C. Hyperkalemia in patients treated with endoradiotherapy combined with amino acid infusion is associated with severe metabolic acidosis. EJNMMI Res. 2018 Feb 27;8(1):17. doi: 10.1186/s13550-018-0370-z.
- Yusufi N, Mall S, Bianchi HO, Steiger K, Reder S, Klar R, Audehm S, Mustafa M, Nekolla S, Peschel C, Schwaiger M, Krackhardt AM, D'Alessandria C. In-depth Characterization of a TCR-specific Tracer for Sensitive Detection of Tumor-directed Transgenic T Cells by Immuno-PET. Theranostics. 2017 Jun 15; 7(9): 2402-2416.
- D’Alessandria C, Braesch-Andersen S, Bejo K, Reder S, Blechert B, Schwaiger M, Bartolazzi A. Noninvasive in vivo imaging and biological characterization of thyroid tumors by immunoPET targeting of Galectin-3. Cancer Res. 2016 Jun 15; 76(12): 3583-92.
- Okamoto S, Thieme A, Allmann J, D'Alessandria C, Maurer T, Retz M, Tauber R, Heck MM, Wester HJ, Tamaki N, Fendlder WP, Herrmann K, Pfob CH, Scheidhauer K, Schwaiger M, Ziegler S, Eiber M. Radiation dosimetry for 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer: Absorbed dose in normal organs and tumor lesions. J Nucl Med. 2017 Mar;58(3):445-450.
- Mendler CT, Feuchtinger A, Heid I, Aichler M, D'Alessandria C, Pirsig S, Blechert B, Wester H-J, Braren R, Walch A, Skerra A, Schwaiger M. Tumor uptake of anti-CD20 Fabs depends on tumor perfusion. J Nucl Med. 2016 Dec; 57(12): 1971-1977.
- Heck MM, Retz M, D'Alessandria C, Rauscher I, Scheidhauer K, Maurer T, Storz E, Janssen F, Schottelius M, Wester HJ, Gschwend JE, Schwaiger M, Tauber R, Eiber M. Systemic Radioligand Therapy with 177Lu Labeled Prostate Specific Membrane Antigen Ligand for Imaging and Therapy in Patients with Metastatic Castration Resistant Prostate Cancer. J Urol. 2016 Aug; 196(2):382-91.
- Mall S, Yusufi N, Wagner R, Klar R, Bianchi H, Steiger K, Straub M, Audehm S, Laitinen I, Aichler M, Peschel C, Ziegler S, Mustafa M, Schwaiger M, D’Alessandria C, Krackhardt AM. Immuno-PET imaging of engineered human T cells in tumors. Cancer Res. 2016 Jul 15; 76(14):4113-23.
- D'Alessandria C, Pohle K, Rechenmacher F, Neubauer S, Notni J, Wester HJ, Schwaiger M, Kessler H, Beer AJ. In vivo biokinetic and metabolic characterization of the 68Ga-labelled α5β1-selective peptidomimetic FR366. Eur J Nucl Med Mol Imaging. 2016; 43 (5): 953-63.
- Neubauer S, Rechenmacher F, Beer AJ, Curnis F, Pohle K, D'Alessandria C, Wester HJ, Reuning U, Corti A, Schwaiger M, Kessler H. Selective imaging of the angiogenic relevant integrins α5β1 and αvβ3. Angew Chem Int Ed Engl. 2013; 52 (44): 11656-9.
- Parisella MG, Chianelli M, D'Alessandria C, Todino V, Mikolajczak R, Papini E, Dierckx RA, Scopinaro F, Signore A. Clinical indications to the use of (99m)Tc-EDDA/HYNIC-TOC to detect somatostatin receptor-positive neuroendocrine tumors. Q J Nucl Med Mol Imaging. 2012 Feb; 56(1): 90-8.
- Gourni E, Demmer O, Schottelius M, D'Alessandria C, Schulz S, Dijkgraaf I, Schumacher U, Schwaiger M, Kessler H, Wester HJ. PET of CXCR4 expression by a (68)Ga-labeled highly specific targeted contrast agent. J Nucl Med. 2011; 52 (11): 1803-10.
- D'Alessandria C, di Gialleonardo V, Chianelli M, Mather SJ, de Vries EF, Scopinaro F, Dierck RA, Signore A. Synthesis and optimization of the labeling procedure of 99mTc-HYNIC-interleukin-2 for in vivo imaging of activated T lymphocytes. Mol Imaging Biol. 2010; 12 (5): 539-46.
- Malviya G, D'Alessandria C, Bonanno E, Vexler V, Massari R, Trotta C, Scopinaro F, Dierckx R, Signore A. Radiolabeled humanized anti-CD3 monoclonal antibody visilizumab for imaging human T-lymphocytes. J Nucl Med. 2009 Oct; 50(10): 1683-91.
- Bartolazzi A, D'Alessandria C, Parisella MG, Signore A, Del Prete F, Lavra L, Braesch-Andersen S, Massari R, Trotta C, Soluri A, Sciacchitano S, Scopinaro F. Thyroid cancer imaging in vivo by targeting the anti-apoptotic molecule galectin-3. PLoS One. 2008; 3(11):e3768.
- D'Alessandria C, Malviya G, Viscido A, Aratari A, Maccioni F, Amato A, Scopinaro F, Caprilli R, Signore A. Use of a 99mTc labeled anti-TNFalpha monoclonal antibody in Crohn's disease: in vitro and in vivo studies. Q J Nucl Med Mol Imaging. 2007 Dec; 51(4): 334-42.
- Soluri A, Trotta C, Scopinaro F, Tofani A, D'Alessandria C, Pasta V, Stella S, Massari R. Radioisotope guided surgery with imaging probe, a hand-held high-resolution gamma camera. IEEE - Nuclear Instruments & Methods in Physics Research Section a-Accelerators Spectrometers Detectors and Associated Equipment. 2007; 583 (2-3): 366-371.
- Annovazzi A, D'Alessandria C, Bonanno E, Mather SJ, Cornelissen B, van de Wiele C, Dierckx RA, Mattei M, Palmieri G, Scopinaro F, Signore A. Synthesis of 99mTc-HYNIC-interleukin-12, a new specific radiopharmaceutical for imaging T lymphocytes. Eur J Nucl Med Mol Imaging. 2006 Apr; 33(4): 474-82.
- Annovazzi A, Bonanno E, Arca M, D'Alessandria C, Marcoccia A, Spagnoli LG, Violi F, Scopinaro F, De Toma G, Signore A. 99mTc-interleukin-2 scintigraphy for the in vivo imaging of vulnerable atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2006 Feb; 33(2): 117-26.
- Signore A, Annovazzi A, Barone R, Bonanno E, D'Alessandria C, Chianelli M, Mather SJ, Bottoni U, Panetta C, Innocenzi D, Scopinaro F, Calvieri S. 99mTc-interleukin-2 scintigraphy as a potential tool for evaluating tumor-infiltrating lymphocytes in melanoma lesions: a validation study. J Nucl Med. 2004 Oct;45(10):1647-52.
Reviews
- Bartolazzi A, Sciacchitano S, D'Alessandria C. Galectin-3: The Impact on the Clinical Management of Patients with Thyroid Nodules and Future Perspectives. Int J Mol Sci. 2018 Feb 2;19(2). pii: E445. doi: 10.3390/ijms19020445.
- Signore A, Chianelli M, D'Alessandria C, Annovazzi A. Receptor targeting agents for imaging inflammation/infection: where are we now? Q J Nucl Med Mol Imaging. 2006 Sep;50(3):236-42.
- Chianelli M, D'Alessandria C, Conti F, Priori R, Valesini G, Annovazzi A, Signore A. New radiopharmaceuticals for imaging rheumatoid arthritis. Q J Nucl Med Mol Imaging. 2006 Sep;50(3):217-25.
- Annovazzi A, Bagni B, Burroni L, D'Alessandria C, Signore A. Nuclear medicine imaging of inflammatory/infective disorders of the abdomen. Nucl Med Commun. 2005 Jul;26(7):657-64.
- Chianelli M, Parisella MG, D'Alessandria C, Corsetti F, Scopinaro F, Signore A. The developing role of peptide radiopharmaceuticals in the study of chronic inflammation: new techniques for novel therapeutic options. Q J Nucl Med. 2003 Dec;47(4):256-69.
Book Chapters
- Signore A, Annovazzi A, Chianelli M, Capriotti G, D'Alessandria C, Biancone L, Bonanno E, Veneziani A, Scopinaro F. Inflammatory Bowel Diseases: the use of radiolabelled cytokines for in vivo evaluation of inflammatory activity. In: Nuclear medicine in the management of inflammatory and infectious diseases - when and how. A. Signore, M. Liberatore, F. Scopinaro, Eds. Springer-Verlag Pbl. Heidelberg 2002.
- Signore A, Chianelli M, D'Alessandria C, Corsetti F, Annovazzi A, De Toma G, Scopinaro F. Diagnostic use of radiolabelled cytokines and chemokines. In: Technetium, Rhenium and other metals in Chemistry and Nuclear Medicine 6. Nicolini M, Mazzi U. Eds. SGE Editoriali Pbl., 2002; p. 637-645.
- Signore A, Annovazzi A, Chianelli M, Capriotti G, D’Alessandria C, Biancone L, Bonanno E, Veneziani A, Scopinaro F. In: Nuclear medicine in the management of inflammatory and infectious diseases - When and How. Signore A, Liberatore M, Scopinaro F, Eds. Springer-Verlag Publ., (in press), 2002; p. 25-30
Additional abstracts and meeting presentations
Additional scientific publications
http://www.ncbi.nlm.nih.gov/pubmed/?term=D%27Alessandria+C(link is external)
Prof. Dr. Calogero D'Alessandria |
Dr. Francesco De Rose |
Dr. Antonia Richter |
Dr. Petra Watzlowik Petra.Watzlowik@mri.tum.de |
Dr. Lisa Russelli |
Michael Herz |
ImmunoPET Precision Imaging with 89Zr-based Antibodies
A primary area of interest of our laboratory is the design, synthesis, characterization, and validation of Zr-89-based antibodies and antibody-derived imaging agents. One of the most fundamental principles in the construction of effective antibody-based nuclear imaging agents is matching the physical half-life of the radioisotope to the pharmacokinetic half-life of the targeting vector. This exigency has led our group to exploit the positron-emitting radiometal 89Zr (t1/2 = 78.41 h) as an effective and versatile long-lived radiolabel for PET imaging. Currently, our laboratory is pursuing a wide variety of projects that employ 89Zr as a radioisotope, as shown in the following examples.
89Zr-Fab2-DFO-Gal3 for new thyroid carcinoma imaging
Thyroid nodules are very common in adults, especially in areas with iodine deficiency. Currently it is often not possible to exclude a malignant nodule by nuclear imaging (radioiodine scintigraphy), ultrasound imaging and fine needle aspiration biopsy, especially in patients with multinodular goiter, because an imaging agent with high and specific uptake in malignant lesions is lacking. A non-invasive imaging test that specifically accumulates in malignant thyroid nodules could substantially reduce the morbidity from thyroid surgeries for benign nodules and reduce healthcare costs.
Galectin-3 (gal-3) is a b-galactoside binding protein expressed in well-differentiated and in undifferentiated thyroid cancer types but not in normal thyrocytes and benign thyroid lesions. We validate gal-3 targeting as a specific method to detect radioiodine non-avid thyroid cancer in thyroid orthotopic tumor models established inoculating papillary (BcPAP) and anaplastic (CAL62 and FRO82-1) thyroid carcinoma cells lines previously characterized via WB and PCR, for gal-3 and sodium-iodide symporter (NIS) expression, in athymic nude mice. An 89Zr-labeled F(ab’)2 anti-gal-3 was generated and characterized for binding versus iodine-125 on 2D and 3D cell cultures. Head-to-head PET/CT comparison of iodine-124 versus 89Zr-DFO-F(ab’)2 anti-gal-3 was performed, followed by biodistribution studies and immunohistochemical analysis for gal-3 and NIS expression. PET/CT imaging showed 89Zr-DFO-F(ab’)2 anti-gal-3 signal associated to the orthotopic implanted tumors only, while no signal was detected in the tumor-free thyroid lobe. Conversely, PET imaging using iodine-124 showed background accumulation in tumor infiltrated lobe, a condition simulating the presence of radioiodine non-avid thyroid cancer nodules, and high accumulation in normal thyroid lobe.
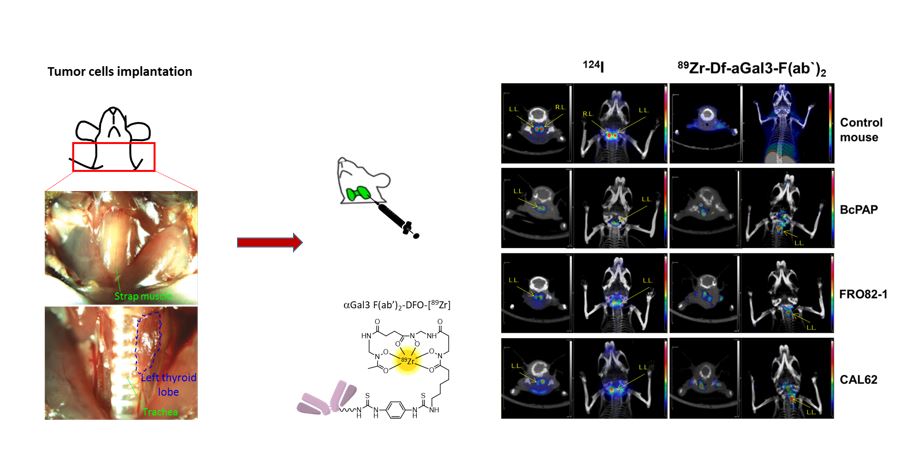
For the clinical translation of this new imaging approach, we have developed, in collaboration with the group of Arne Skerra (Biochemistry Depart. TUM), a humanized Fab’ anti gal-3 presenting a prolonged plasma half-life and tumor accumulation via PASylation technology.
89Zr-Fab2-DFO-Gal3 to visualize atherosclerotic plaques remodeling
Atherosclerosis is a multifactorial chronic inflammatory disorder characterized by a disturbed equilibrium of immune responses and lipid accumulation, leading to plaque development. Most atherosclerotic plaques remain clinically asymptomatic, though some undergo a series of changes that cause life-threatening complications. Visualization of atherosclerotic plaques and in particular identification of the high-risk lesions prone to rupture in vivo is a challenging objective with the ultimate goal to develop pharmacological and/or therapeutic strategies to prevent lethality. Molecular imaging offers potential opportunities to develop diagnostic approaches to assess the pathobiology of atherosclerotic plaques. Improved understanding of the molecular and biological processes has stimulated the development of imaging probes, which may aid to identify high-risk atherosclerotic lesions and to apply individually tailored interventions. Monocyte-derived macrophages are major inflammatory cells associated with atherosclerotic lesions and are recognized as key pathophysiologically important immune cells. Therefore, macrophages are gaining attention as imaging targets in atherosclerosis. Galectin-3 (Gal3) is a member of the lectin family, which is known to be involved in multiple aspects of inflammatory cell pathology, and in particular is a constitutive marker of activated macrophages. Overexpression of Gal3 in the aorta of hypercholesterolemic animals and in human atherosclerotic lesions has been reported, suggesting a direct involvement of this multifaceted protein in the pathophysiology of the disease. Gal3 has therefore been proposed as a potential target for non-invasive molecular imaging of atherosclerosis and in particular plaque vulnerability.
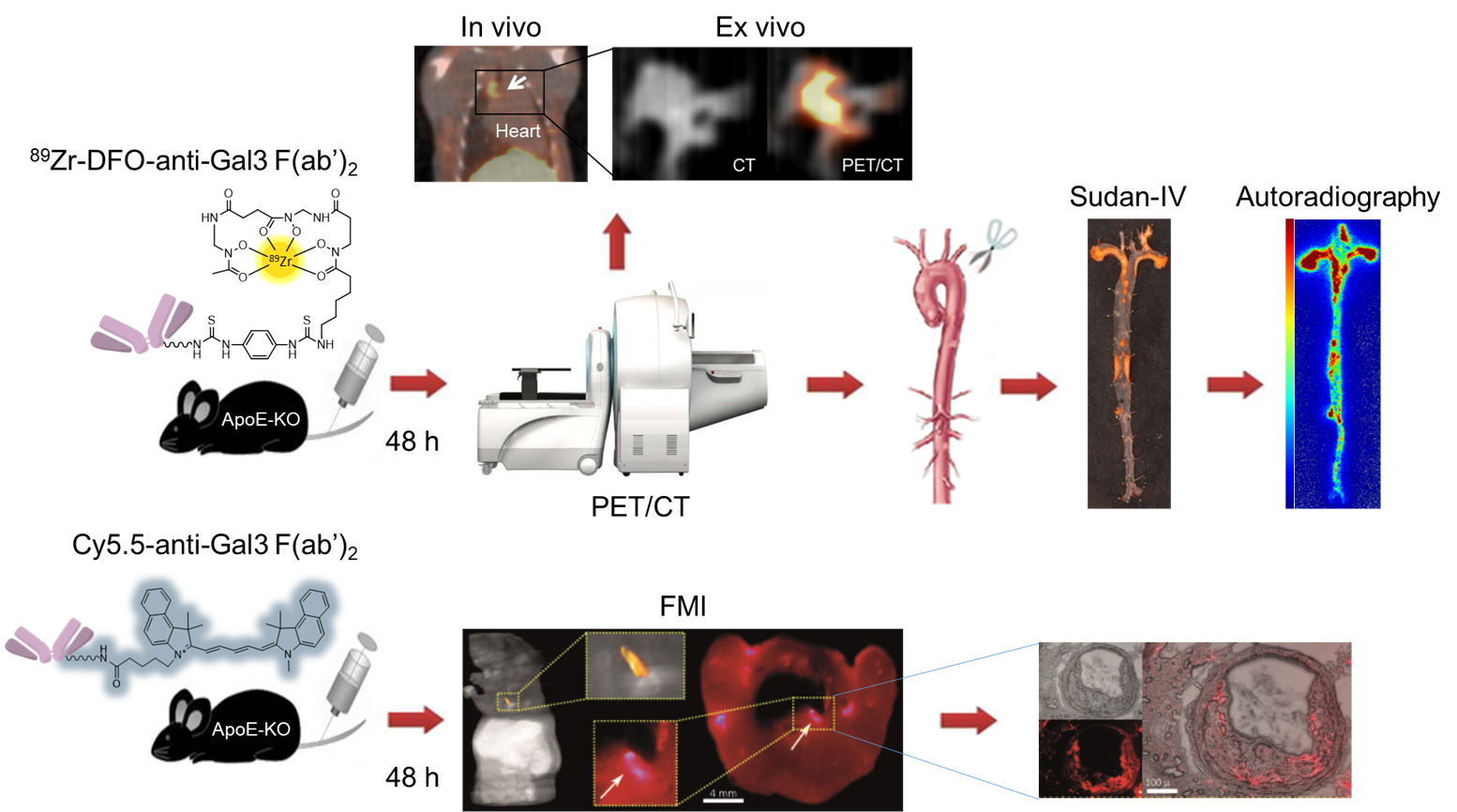
Figure legend: Multimodal imaging approach to confirm the expression of Galectin-3 in atherosclerotic plaques in remodeling and macrophages infiltration via 89Zr-Dfo-F(ab’)2 anti-Gal3 and fluorescent molecular tomography.
Using zirconium-89 labeled Gal3-F(ab’)2 mAb in combination with fluorescent molecular tomography imaging (in collaboration with Vasilis Ntziachristos), we have shown that nuclear imaging has the potential to visualize specific biological activities associated with plaque progression and/or instability, and that Gal3 expression in atherosclerotic plaques may be used as a novel biomarker to identify patients at high risk of cardiovascular events or to monitor the impact of treatment aimed at plaque stabilization.
89Zr-based Antibodies for Imaging T cell distribution and homing
Genetic modification of effector T cells by T-cell receptors (TCR) specifically recognizing tumor-associated peptide ligands is a highly attractive novel strategy for the treatment of diverse malignant diseases. However, in order to understand T cell trafficking, expansion and functionality in preclinical models, the development of non-invasive and highly sensitive cell-tracking technologies is necessary. Moreover, clinical translation of such technologies would further provide possibilities to improve this therapeutic approach in humans and may provide options for directed depletion.
To this purpose, in collaboration with Angela Krackhardt (III Medical Department of MRI-TUM), we have taken advantage of using an alloreactive TCR with high specificity for the leukemia associated differentiation antigen myeloperoxidase (MPO) which is used for genetic modification of central memory T-cells (TCM). To monitor TCR-transduced T cells in vivo, we used Zirconium-89 labeling of an anti-murine TCR antibody (TCRmu), which binds to the murinized part of the TCR beta chain of the introduced TCR. We could detect specific 89Zr-TCRmu binding to TCR-transduced TCM in vitro and those cells demonstrated functionality.
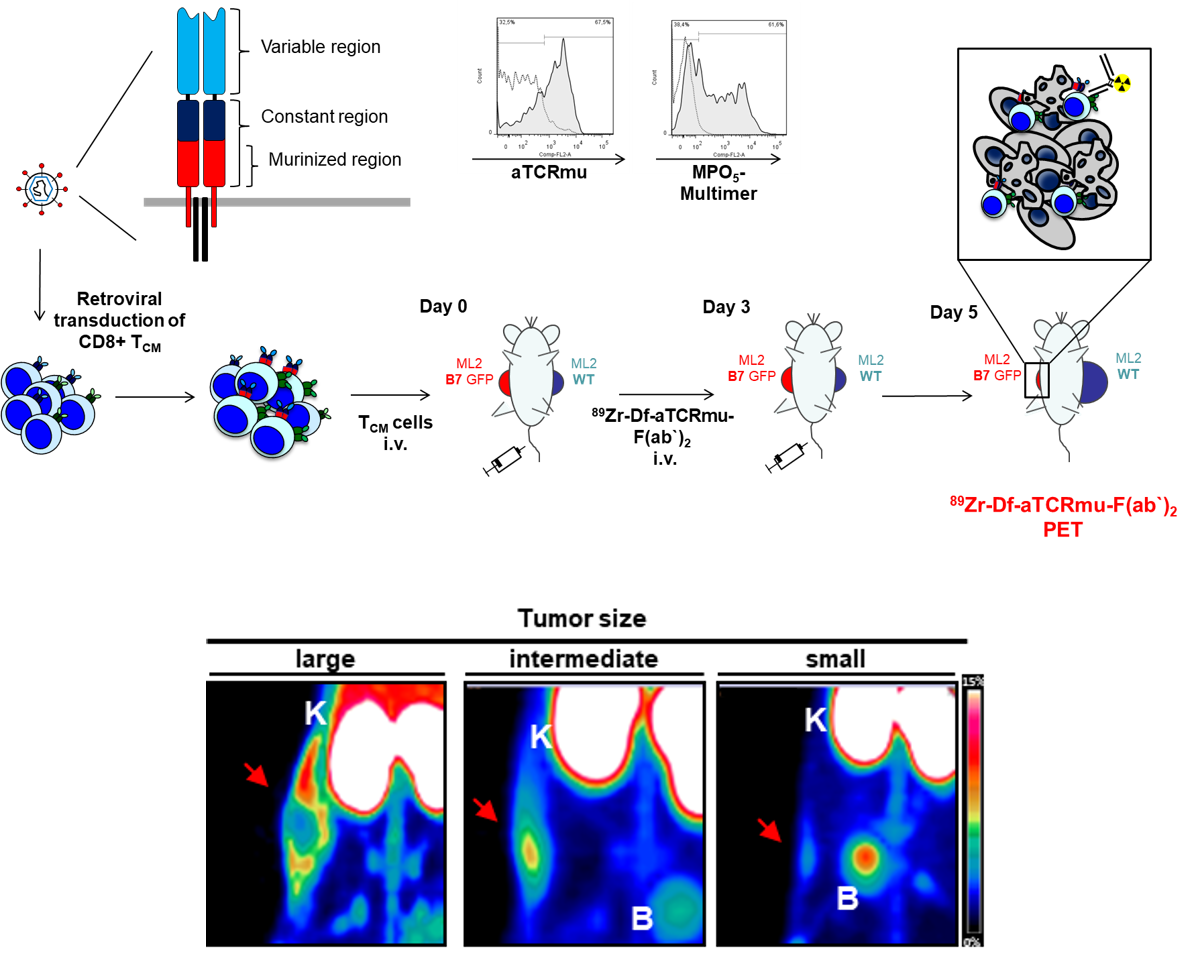
Figure legend: Scheme of production of TRC-transgenic T cells and tumor size-driven reactive T-cell infiltration visualized by 89Zr-Fab2 anti-TCRmu tracer.
A xenogenic mouse model was established using immunocompromized mice injected subcutaneously with the AML-cell line ML2. After tumor inoculation, we injected intravenously TCR-transduced human T cells and PET/CT imaging was performed at different time points post intravenous injection of 89Zr-labeled Fab2 fragment of the antibody which recognizes the murinised portion. In vivo application demonstrated a strong signal on the tumor site where TCR-transgenic T cells were accumulating, with a signal distribution directly correlating with the number of T cell infiltrating the tumor and with the tumor size, but no signal when untransduced T cells or PBS were used. Using this experimental approach, we have proved that a non-invasive imaging approach for tracking specifically human TCR-engineered lymphocytes in vivo is feasible. This Fab2-fragment based PET imaging modality may be useful to monitor adoptively transferred T cells transduced with diverse TCR in murine models. Moreover, this imaging modality may be suitable for clinical translation.
89Zr-based molecules as tracers for Imaging heat-shock protein 70 in tumors
The search for suitable PET tracers for tumor-specific detection in early stages and monitoring of therapy responses can improve clinical outcome. Clinically applied tracers frequently address metabolic tumor features (e.g. [18F]-FDG) or target molecules that are overexpressed in tumor cells, but are also displayed on the plasma membrane of normal cells. Consequently, these tracers might show suboptimal tumor specificity and false positive signals in non-neoplastic tissues. Therefore, novel imaging approaches focus on tracers targeting epitopes that are exclusively present on tumor cells. The membrane-bound form of the major stress inducible 72kDa heat shock protein 70 represents such a tumor-specific target. Hsp70 is expressed in the cytosol of all nucleated cells where it fulfils a variety of chaperoning functions, such as folding and assembly of nascent polypeptides, refolding of denatured proteins, as well as regulation of protein transport across membranes. The majority of human tumors of different entities is characterized by a constitutive overexpression of Hsp70 which supports tumor progression, survival, metastatic spread and resistance to therapy.
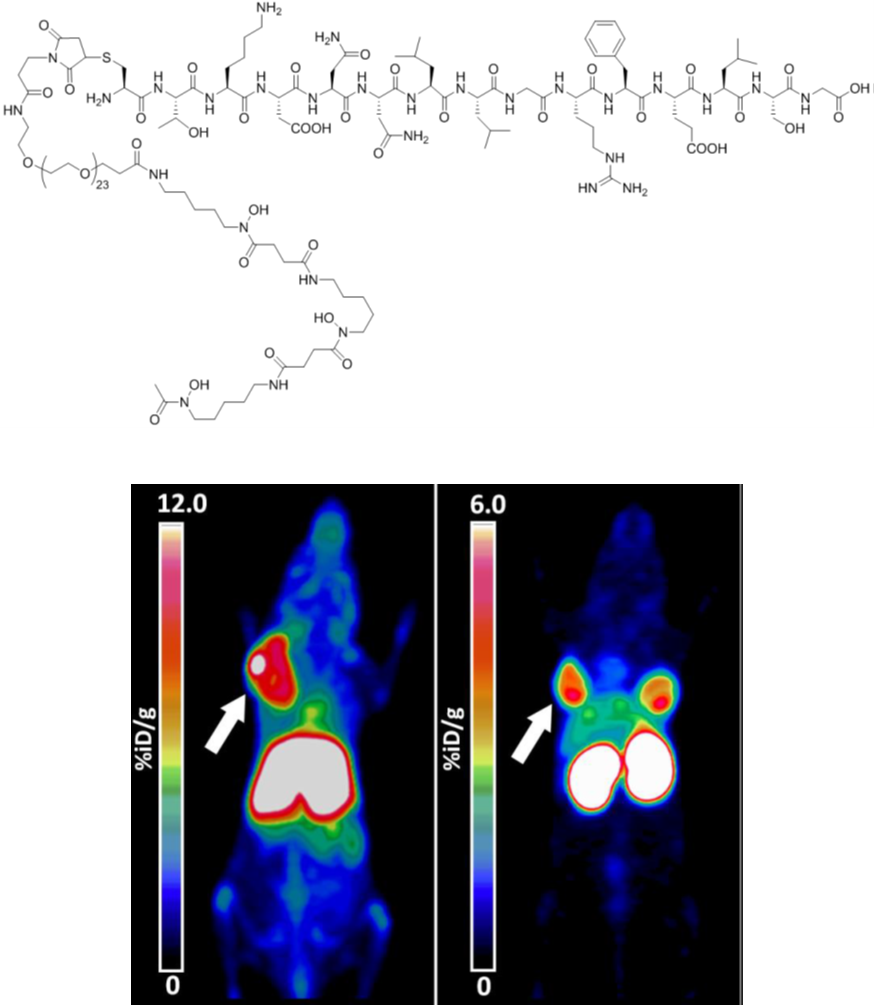
Figure legend: Representative pseudocolor PET scans of cmHsp70.1-DFO[89Zr] in a living mouse bearing sc implanted 4T1 wt tumor, 72h after iv administration (left), and of a mouse bearing sc implanted 4T1 (left shoulder) and CT26 (right neck) tumors, 24h after iv TPP-PEG24-DFO[89Zr] injection (right).
Preclinical therapeutic approaches using cmHsp70.1 mAb for targeting mHsp70 on tumor cells revealed an ample tumor-specific binding in vivo, that results in a potent activation of the hosts´ antibody dependent cellular antitumor immune response. However, due to the large size (150kDa) and the immunogenic potential, antibody-based PET probes, investigated in collaboration with Gabriele Multhoff (Radiation oncology dept. MRI-TUM), exhibit certain limitations for imaging purposes including unfavorable biodistribution kinetics caused by long blood circulation times and slow tumor uptake, high accumulation in the liver which increases the risk of hepato-radiotoxicity, and Fc receptor based off-target effects. For in vivo imaging applications, we chose to use a smaller molecule, such as the 14mer peptide TPP that mimics properties of the oligomerization domain of Hsp70 and enables a tumor-specific targeting via mHsp70. This approach shows numerous advantages over antibody including short circulation periods, fast body clearance, favorable biodistribution, improved ingress into solid tumors and highly efficient tumor cell penetration capabilities.
Development of new chelators for PET radiometal complexation
New ligands for small temperature sensitive 68Ga-radiopharmaceutical
The widespread clinical application of 68Ge-based radioisotope generators (t1=2 (68Ge)=270.8 days) for the production of the PET isotope 68Ga (t1=2=67.71 min, Eb+,max=1.89 MeV, 89% decays through positron emission), along with the favorable properties of the isotope (i. e. sufficient half-life for producing tracers that expose the patient to a minimal radiation dose), has boosted research activity aiming to design effective, specific and safe 68Ga-based radiopharmaceuticals. A 68Ga-based radiopharmaceutical consists of a thermodynamically stable and kinetically inert GaIII complex linked to a specific vector, most often represented by peptides or pseudopeptides. Ga3+ ions are typically complexed at acidic pH to avoid the formation of Ga(OH)3, by a chelator having at least 6 donor atoms (N- or O-donors) that is able to wrap around the metal ion and prevent transmetallation reactions with endogeneous metal ions (Cu2+, Zn2+, Ca2+) or ligand exchange reactions with proteins such as transferrin. An efficient 68Ga-labeling reaction, that does not require high temperatures (i. e. 958C as for DOTA-based systems) represents a very attractive opportunity when producing fragile, temperature sensitive macromolecular tracers. In this context, a polyaminopolycarboxylate heptadentate ligand based on a 1,4-diazepine scaffold (AAZTA=6-amino-6-methylperhydro-1,4-diazepinetetraacetic acid, Scheme 1) has been thoroughly studied as chelator for 68Ga, 111In and 177Lu for application in PET, SPECT and radionuclide therapy. With the aim to further improve the performance of the AAZTA chelator for labeling temperature sensitive molecules, we designed and investigated in collaboration with Lorento Tei (UNIPO, Italy) a new ligand containing a cyclohexane ring fused onto the seven-membered diazepine ring, raising its structural rigidity.
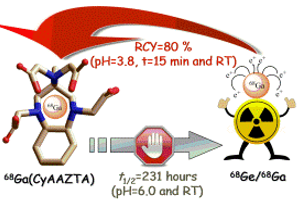
Figure legend: Cartoon showing the coordination of 68Ga from CyAAZTA chelator suitable for temperature sensitive molecules.
The presence of the trans-1,2-diaminocyclohexyl group in the newly synthesized ligand CyAAZTA led to the formation of a structurally rigid GaIII complex having good thermodynamic stability and kinetic inertness. These characteristics of Ga(CyAAZTA)- is well suited for 68Ga PET studies as decomposition takes considerably longer than the radioactive half-life of the 68Ga isotope. The complexation of 68Ga with CyAAZTA performed at RT and pH 3.8 within 15 min provide a yield of >80% and at 90°C with a near-quantitative yield (in 5 min), with high stability in HS and DTPA excess at 37°C. The potential of the proposed chelator is strenghtened by the wide spectrum of possible CyAAZTA-based bifunctional chelators that can be prepared by adapting procedures used for the syntheses of functionalized AAZTA ligands. Therefore, room temperature labelling can be of interest for temperature sensitive molecules and labelling at higher temperatures may be advantageous for reaching higher specific activity in peptide radiopharmaceuticals.
Chelators for Al18F labelling of biomolecules for PET-diagnosis
Pentadentate chelators able to stably complexed [Al18F]2+, thanks to their rigid structure, and to be marked with 18F very fastly at room temperature. The direct labelling at low temperature allows the diagnosis of several pathologies, thanks to the further conjugation of the chelators with sensible biomolecules, as proteins, anticorporal fragments, nanobodies, scFv.
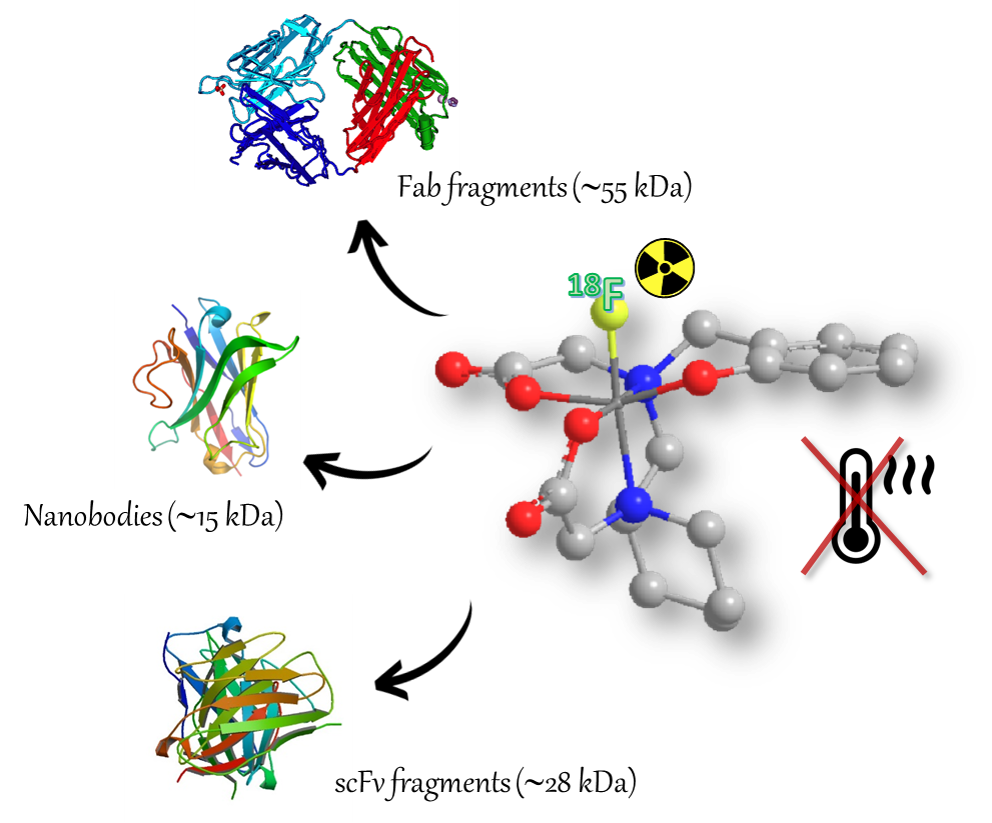
Figure legend: Cartoon showing diverse possible application for temperature sensitive molecules radiolabeling using the new pentadentate chelator complexing [Al18F]2+.
The presence of heterocyclic or polycyclic structures and of five donator atoms, two neutral N-amminics and three negative O-carboxylic or phenolic, allows to obtain a fast and stable complexation also at room temperature. Moreover, the labelled target demonstrated a good stability in physiological conditions and in vivo, a low 18F accumulation into the bones and a fast hepatobiliary depletion. These prerogatives allow the development of bi-functional chelators able to conjugate a wide range of specific biomolecules for the pathologies determination. The Al18F labelling technique could allow the early diagnosis of oncological diseases and/or the identification of specific pathology at the initial stage. A wide number of applications of the bi-functional chelators with several selective biomolecules can be envisaged to identify pathologies detected with PET-imaging technique.
Semi rigid chelators for 89Zr labelling of biomolecules
Due to the fact that immunoPET has become the method of choice for imaging not only tumors but also immune cells, immune checkpoints, and inflammatory processes, the radiochemistry of Zr-89 and complexation strategies to use this radioisotope has driven the design and development of new chelators. We have recently developed and investigated hexadentate chelators for zirconium-89 starting from the heterocyclic structure of the triamine AMPED by insertion of three bidentate N-methylhydroxamate coordinating groups. The initial hypothesis was that longer pendant arms would better coordinate the Zr-89 isotope, leading to a more stable complex over time. A functionalized derivative of the most promising one was also synthesized and characterized to allow the conjugation with a humanized antibody and the subsequent 89Zr labeling.
Figure legend: Coordination scheme of 89Zr from the semi rigid AMPED-based chelator.
We selected the chelator with longer arms, AAZTHAG, as the best complexing agent for 89Zr presenting a stability of 86.4 ± 5.5% in human serum (HS) for at least 72 h. An activated ester functionalized version of AAZTHAG was synthesized to allow the conjugation with biomolecules such as human anti-HER2 monoclonal antibody Trastuzumab (Tz), as a proof of principle test. The final 89Zr labeled compound was characterized via radio-HPLC and SDS-PAGE followed by autoradiography, and its stability in different solutions confirmed for at least 4 days.
Cooperations partners & Founding




